The concept of heat and temperature are investigated together in science which is moderately related but not alike. The terms are extensive due to their wide usage in our daily life. There is a fine line that separates heat from temperature, in the sensation that heat is considered a form of energy, though temperature is a measure of energy.
The difference between heat and temperature is small but significant; heat is the molecular motion’s overall energy, while the temperature is the average energy of the molecular motion. So, let’s have a look at the explanation provided below.
What is Heat?
In order to find the difference between heat and temperature, let us first introduce heat. Heat is described as the total amount of energy available in the body. Heat is a kind of energy that passes from one source to another one and is also transmitted further to other objects because of their temperature difference. Of course, heat moves from hotter materials to cooler ones.
It is the total amount of kinetic in addition to potential energy available in the object. Heat is measured in an energy unit termed Joules.
Heat is also described as transient energy while it is continuously transmitted. Once it enters a mass, it is no longer called heat energy. At this point, it is called internal energy. Heat is a necessary energy component for living. It is the vital energy that is required for human survival too.
The are many sources of heat energy, but the primary source of heat energy is the Sun. Other sources of heat energy are electricity, fossil fuels, and nuclear reactions. The level of heat grows when the molecules in the body tend to move faster within the body. That is when the molecules are in more kinetic energy.
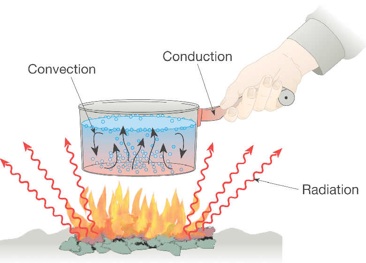
The heat energy can be transferred in three different ways: conduction, convection, and radiation.
- Conduction: Heat transfer between molecules that are in direct contact with each other without particles’ movement.
- Convection: The transfer of heat that occurs due to particles’ motion from one position to another is convection.
- Radiation: When the heat is carried through a medium or vacuum, wherein space in between is not heated up.
Misconceptions about Heat
Before go for difference between heat and temperature, see misconceptions about heat. Heat is the same as work in that both describe a means for the transfer of energy. Neither heat nor work is an inherent property of energy. Neither heat nor work is an innate property of a system; that is, we cannot say that a system “contains” a definite amount of heat or work. Alternately, we say that it can carry a certain amount of energy as heat or work under certain designated conditions.
Some of the confusion about the accurate meaning of heat results from the extensive usage of the term. Often heat is utilized when what is really meant is temperature or possibly internal energy. When we hear about the heat in association with the weather, or when cooking instructions show “heat at 320 degrees” it is the temperature that is being addressed. On the other side, we also find references to the “heat generated” by the brake linings of an automobile or by quickly rubbing the palms of your hands together. In this state, it is often internal energy that is implied. A clue to the appropriate usage comes from the description of heat: When you rub your hands together, they do work on each other, thereby enhancing their internal energy and increasing their temperature. This excess energy can then be carried to the environment as heat since the hands are at a higher temperature than the environment.
Unit of Heat
Because heat is a class of energy, its units are those of energy, specifically, the Joule (J) in the SI system. Before it was accepted that heat is a form of energy, other units were allocated to it. In some cases, these units, particularly the calorie (cal) and the British thermal unit (Btu), are still practiced today. They are linked to the Joule according to:
1 Btu=1055 J
and
1 cal =4.186 J.
The “calorie” in common usage as a measure of nutrition (Cal) is, in fact, a kilocalorie; which is
1 Cal = 1000 cal = 4186 J.
The Btu is still usually found to estimate the ability of an air conditioner to transport energy (as heat) from a container to the outside atmosphere. A typical air conditioner rated at 10,000 Btu/h can, therefore, transfer about 10 7 J from a room each hour and carry it to the outside environment.
The Mechanical Equivalent of Heat
In history, when the calorie was individually defined as a unit of heat, it was required to determine an empirical correlation between the calorie and the Joule. This was first arranged by James Joule in 1950 in an experiment to ascertain the mechanical equivalent of heat. The final result of Joule’s experiment and others that followed presented for nearly 100 years a conversion among the Joule and the calorie. Following the approval in 1948 of the Joule as the SI unit of work and heat, in J, this conversion factor has lost its significance in Joule’s time. Nevertheless, Joule’s work is still remarkable for the skill and originality of his experiments, for its accuracy (Joule’s results differ by only 1 % from the SI defined the connection between the Joule and the calorie), and for the way it presented in showing that heat, like work, could the property be considered as a means of transferring energy.
What are the Primary Sources of Heat?
- The Sun is the most essential source of heat. Sun’s heat arrives at the Earth in the form of radiations. Solar radiations hold the Earth’s environment warm at a proper temperature for the survival of life.
- We keep our body warm and lively by the heat generated from the food while its metabolism in the body cells.
- Heat is also generated by burning fossil fuels(wood, oil, coal, gas, etc.). We prepare food and warm our apartments with the heat provided by burning wood and natural gas. The heat generated by burning coal and oil etc. is utilized to generate electricity in thermal power stations.
- Electricity is also utilized to generate heat.
- Heat is received from nuclear reactions like Fusion and Fission.
- Biomass gas is a source of heat.
What is Temperature?
A physical property of a substance that defines the amount of heat content in a substance is identified as temperature. It explains the substance’s hotness or coldness, therefore, corresponding to the particles’ average kinetic energy.
We know that cold and hot are non-scientific terminologies, but both of them hold colossal significance when the word temperature is discussed. We have already explained that a substance with more heat is hot while the cold one has more limited heat. This is the basis for defining temperature, which deals with the particles’ molecular motion in a substance; the degree of coldness or hotness is considered.
Unlike heat, the temperature measurement is detached of mass or type of particle, as it is an average measurement thus relevant to particles’ motion. A body with a high temperature implies that there is a high energy vibrational flow of the molecules.
Consider an instance of two separate vessels, one of 2ml and the other of 4ml, wholly saturated with water. Even if the two vessels are at an equal temperature, the vessel with more water contains more heat than the one with less water because more water will have more thermal energy, notwithstanding having the same temperature. Now, we can go for difference between heat and temperature.
Critical Difference Between Heat and Temperature
There are some differences between heat and temperature. Such as:
- The heat present in a substance implies the total amount of energy (i.e. the total of potential and kinetic energy) of the molecules. While the temperature of the substance is connected with the average kinetic energy of the molecule.
- For two objects connected, the total heat energy will be the total of each item’s thermal energy content. Nevertheless, for two bodies in contact with various temperatures, the final temperature will value the two values.
- Heat is defined in terms of Joule, while sometimes also defined in calories. However, the temperature is regularly measured in Kelvin, while the other measuring units are Fahrenheit and Celsius.
- The heat available in a substance is measured by the calorimetry principle; therefore, a calorimeter is a device that calculates heat content. In contrast, the temperature is measured using a thermometer.
- The heat in a substance depends on the speed with which the particles are disturbing, type of particle, mass, etc. In contrast, the temperature dependency is directly concerned with the molecular movement in the substance.
- The heat in a substance is indicated as symbol Q, while the temperature is displayed by symbol T.
- Heat is fundamentally a kind of energy, while the temperature is a means of that energy.
- Heat is measured as the output of several particles and each particle’s energy content. On the opposite, temperature measurement is correlated with how fast or slow the molecules are transferring with the heat change.
Conclusion
We talked about difference between heat and temperature. Both these concepts are of prime significance in science. Heat energy entirely shows the total number of particles existing in the body. However, the temperature is just a measure of the average energy of the particles of the material.
Heat is a great source of energy for human survival. If it goes high, it is difficult and also challenging if the heat comes down also. It is the temperature that will say what degree of heat is suitable for humanity.
As such heat does not give a clear picture of survival except for the exposure. Heat is directly connected to the body’s mass, while the temperature is directly related to the kinetic energy of the molecules. To find out more, you can watch here.
Buy Equipment or Ask for a Service
By using Linquip RFQ Service, you can expect to receive quotations from various suppliers across multiple industries and regions.
Click Here to Request a Quotation From Suppliers and Service Provider
Read More on Linquip
- What Is Conduction Convection and Radiation? (With Example)
- At What Temperature is a Heat Pump Not Effective? Easy Answer
- What Are 10 Common Causes of Overheating?
- Difference Between 2 Stroke and 4 Stroke
- Types of Heat Exchangers: An Introduction to All Essential about Specifications
- Difference between General Relativity and Special Relativity
- Difference Between Grounding and Earthing
- Difference Between Hydraulics and Pneumatics
- How to Calculate the Efficiency of Heat Exchangers?